Abstract
Aplastic anemia (AA) is a bone marrow failure (BMF) disorder characterized by pancytopenia and hypocellular marrow. In cases that are not constitutional or due to environmental causes, an immune-mediated etiology is inferred from the efficacy of immunosuppressive therapies and extensive work implicating cytotoxic CD8+ T cells in bone marrow destruction. To study AA experimentally, BMF is induced in mice with major histocompatibility complex-mismatched C67BL/6 (B6) ⇒ CByB6F1 lymph node (LN) cell infusion following sublethal total body irradiation (TBI). Following recent FDA approval of the JAK 1/2 inhibitor ruxolitinib (RUX) for graft-versus-host disease (GVHD), and based on its suppressive effect on T cell activation and inflammatory cytokine production, we studied RUX in our murine BMF model. Our results demonstrate that RUX attenuated BMF effectively by suppressing T cell activation and proliferation.
Regulatory T cells (Tregs) are a heterogeneous subpopulation of cells that prevent autoimmunity by suppressing autoreactive T cells. Murine Tregs are identified by cell surface expression of the T-cell co-receptor CD4 and the activation marker CD25 and intracellular expression of the transcription factor FoxP3. As a master regulator of Treg development, FoxP3 associates with other transcription factors to induce the Treg functional identity. When assessing Tregs in a laboratory setting, cell fixation and permeabilization are required for antibody binding to FoxP3 due to its intranuclear location, preventing isolation of viable Tregs. To isolate intact Tregs, CD4 and CD25 double positivity alone is conventional, but some prior studies evaluating JAK inhibition used only CD4+FoxP3+ and excluded CD25. Thus, there is lack of consensus in definition of these important immunoregulatory cells in different immune contexts.
Our studies revealed a RUX-mediated decrease in T-cell activation, evidenced by decreased CD25 surface expression in both CD8+ [30.5±2.71 (BMF n=7) vs 9.48±1.30 (RUX, n=10), p<0.0001] and CD4+ [33.76±2.40 (BMF n=7) vs 2.29±1.08 (RUX, n=10), p<0.0001] T cells. Concurrently, FoxP3 expression was increased [7.61±2.16 (BMF n=7) vs 16.12±0.94 (RUX, n=10), p<0.01] in CD4+ T cells, indicating a higher quantity of Tregs. We therefore postulated that, in RUX treatment, CD25 expression primarily reflects activation status rather than marking induction of Tregs. We sought to identify other surrogate surface markers for FoxP3, in order to enable Treg identification and isolation to evaluate immunoregulatory function.
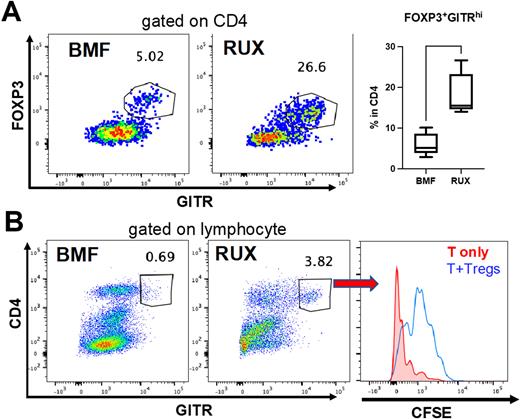
We investigated the surface markers GITR, CD45RB, and CD127, which have been previously described in association with Tregs, to characterize Tregs in BMF control and RUX-treated BMF mice. We observed strong co-expression of GITR and FoxP3 (Figure 1A), suggesting that CD4+GITRhi expression identified the Treg phenotype. CD4+FoxP3+ cells were consistently GITRhi but had lower CD25 expression in RUX-treated BMF samples than in BMF controls, implying that changes in CD25 expression from RUX treatment may render CD25 an unreliable marker of Tregs in this context. Tregs identified by both CD4+FoxP3+ and CD4+GITRhi showed co-expression of CD45RB and CD127. The proportions of cell populations that were CD45RB and CD127 double-negative and double-positive unexpectedly differed, according to whether BMF mice were untreated or RUX-treated, suggesting possible phenotypic changes in Tregs induced by RUX.
To assess the function of Tregs in the spleen of RUX-treated BMF samples, we used flow cytometry to sort Tregs based on expression of CD4+GITRhi, and we co-cultured them with activated CFSE-labeled T cells in vitro at a 1:1 ratio for 5 days. A CFSE assay demonstrated slower CFSE dilution among T cells co-cultured with Tregs compared to those without Tregs (Figure 1B). These isolated Tregs inhibited both CD4+ and CD8+ T cell proliferation, but CD8+ T cells to a greater extent, and similarly to Tregs isolated from healthy mice.
Murine Tregs can be reliably identified using CD4+GITRhi in immune contexts involving altered T-cell activation. We demonstrate phenotypic changes in the proportions of Tregs expressing surface marker combinations after RUX treatment.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.